As digital innovations continue to improve healthcare outcomes, Payers and health care organisations recognize the need for a standardized methodology to identify effective digital solutions that both are cost-saving and suitable for use from clinical, IT, and patient perspectives.
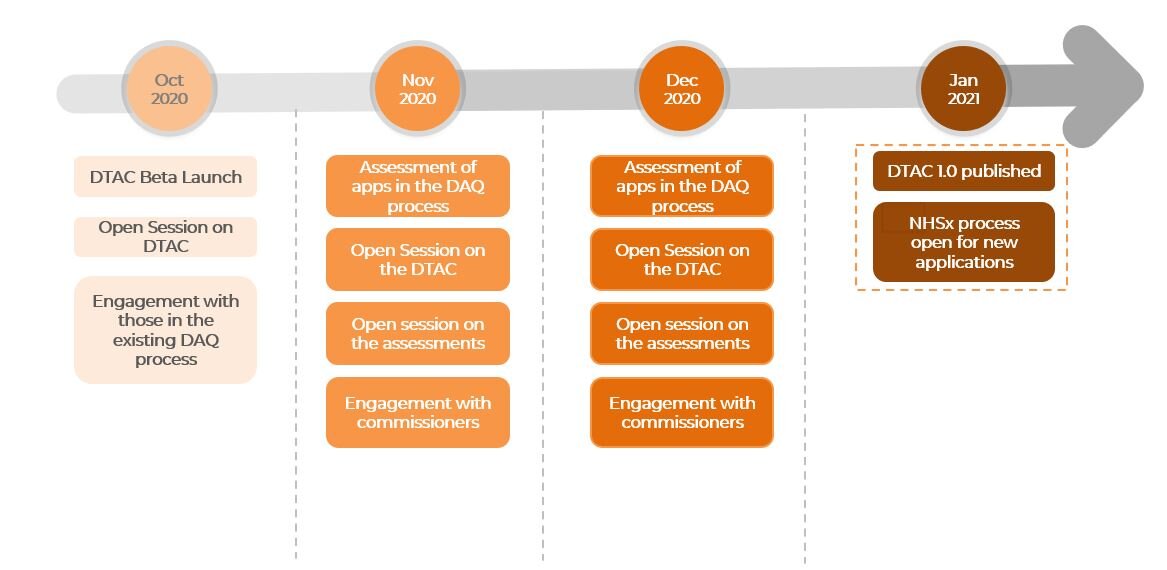
Recently in Oct 2020, the NHSx, UK’s National Health Service arm in charge of digital and data, launched a digital technology assessment criteria (DTAC) beta. A new version, to be confirmed in Jan 2021, will be used to assess apps for inclusion in the NHS Apps Library, a list of trusted apps validated by the NHS.
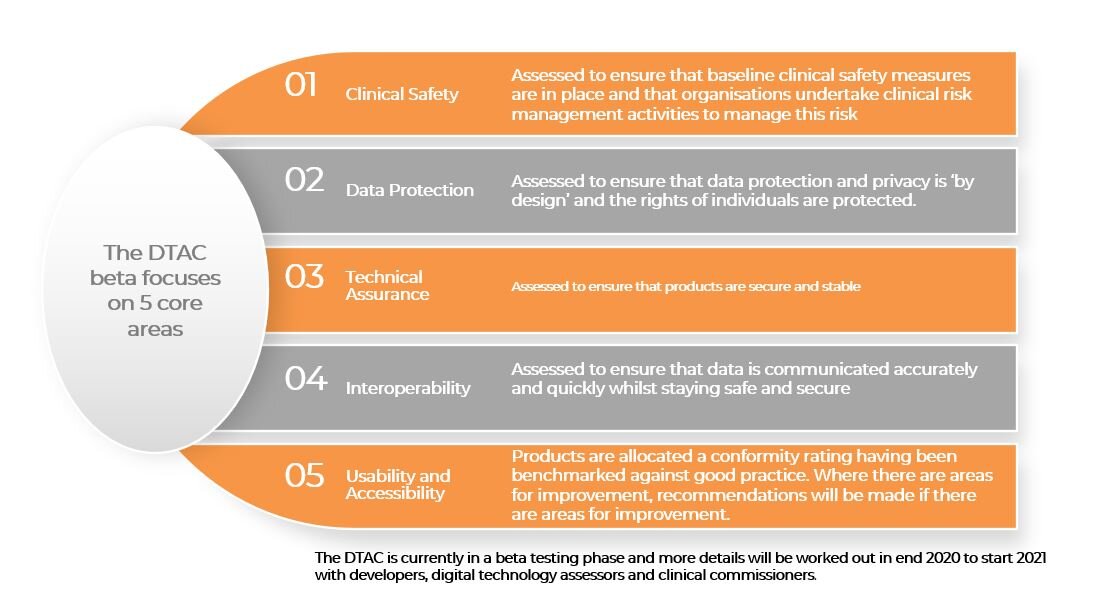
The DTAC beta includes basic user aspects of digital solutions including clinical safety, data protection, technical assurance, interoperability, usability, and accessibility. It focuses on detailing the specific requirements that must be evidenced before digital health technologies can be used directly by patients or procured safely by commissioners and the wider health and care system.
The DTAC will also replace the existing Digital Assessment Questionnaire (DAQ) and Digital Assessment Portal (DAP) as assessment criteria for inclusion in the NHS Apps Library.
Whilst still in beta phase now, the finalised NHSx DTAC is targeted to be open for new applications by Jan 2021.
DTAC: Digital Technology Assessment Criteria, DAQ: Digital Assessment Questionnaire
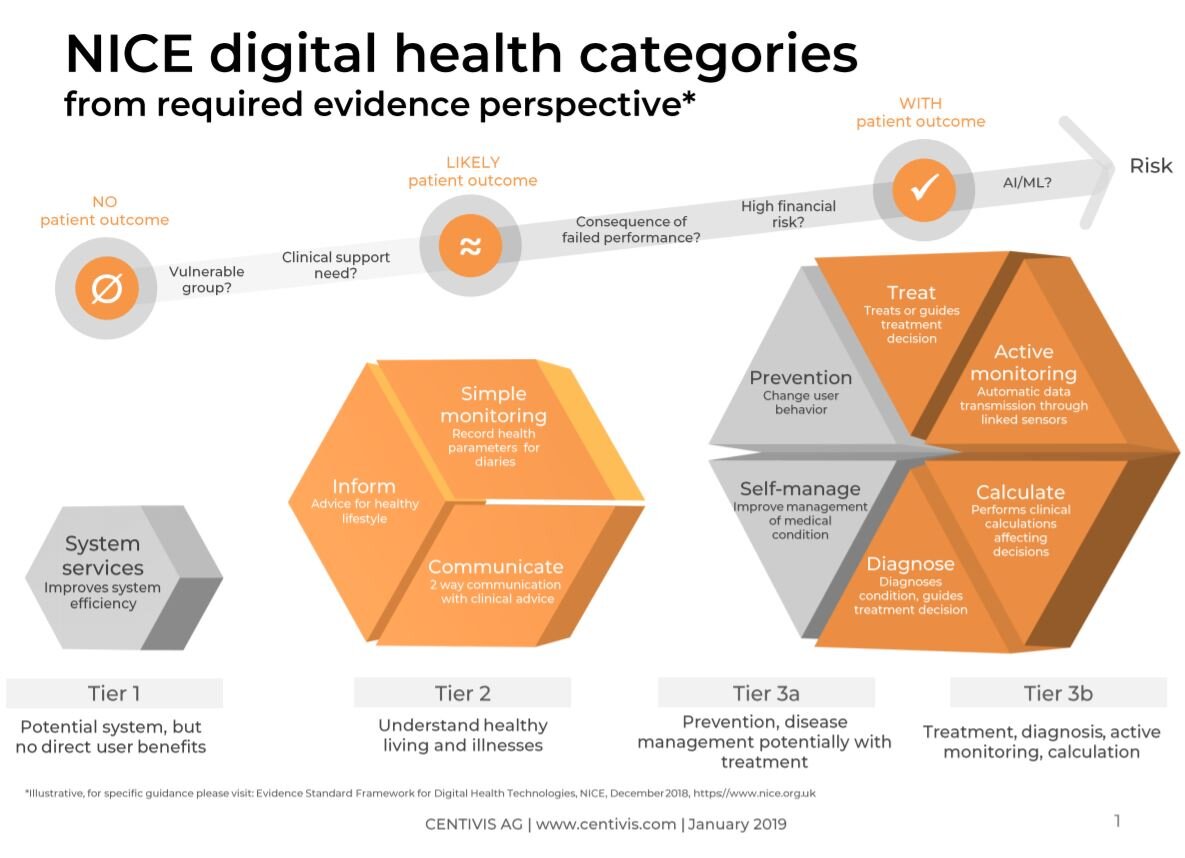
Compared with the National Institute for Health and Care Excellence’s (NICE) Evidence Standards Framework for digital health technologies, the NHSx DTAC seems to assess more basic user safety, technical aspects. NICE’s criteria assesses the technology’s effectiveness and economic impact evidence based on which risk tier it falls into. (For more information on evidence required per tier, see here)
Centivis is monitoring the situation for more updates.